MES
Technology Stack
* The below logos are copyrighted and owned by their respective owners / organizations.












* The logos are copyrighted and owned by their respective owners / organizations.
MES
HITPHAMS (Pharma MES) –Hitachi Pharmaceutical Manufacturing Execution System is a packaged system designed to manage pharmaceutical and biotech manufacturing compliant with cGMP, EC-GMP, and J-GMP.
CIC is a high-productivity and high-quality software development company that established a vehement partnership with HITACHI Limited, a well-known Japanese multinational conglomerate that provides sophisticated business and consumer solutions that transform business processes. This consummate partnership platform supports pharmaceutical companies by implementing HITPHAMS to streamline and tighten their manufacturing operations to implement stricter manufacturing and quality controls according to global requirements.
HITPHAMS will convert the current Pharma manufacturing paper works electronically as eBMR, eBPR, and eLog Book.
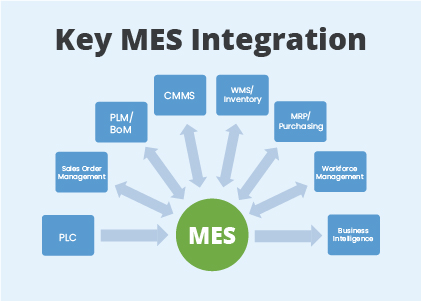
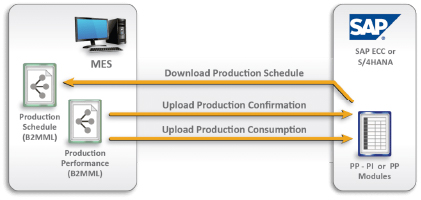
HITPHAMS connects, monitor, and control complex manufacturing systems and data flow on the factory floor. And it will communicate with all GMP systems like ERP (SAP), LIMS, DMS, CLS, QMS, SCADA, and eDMS.
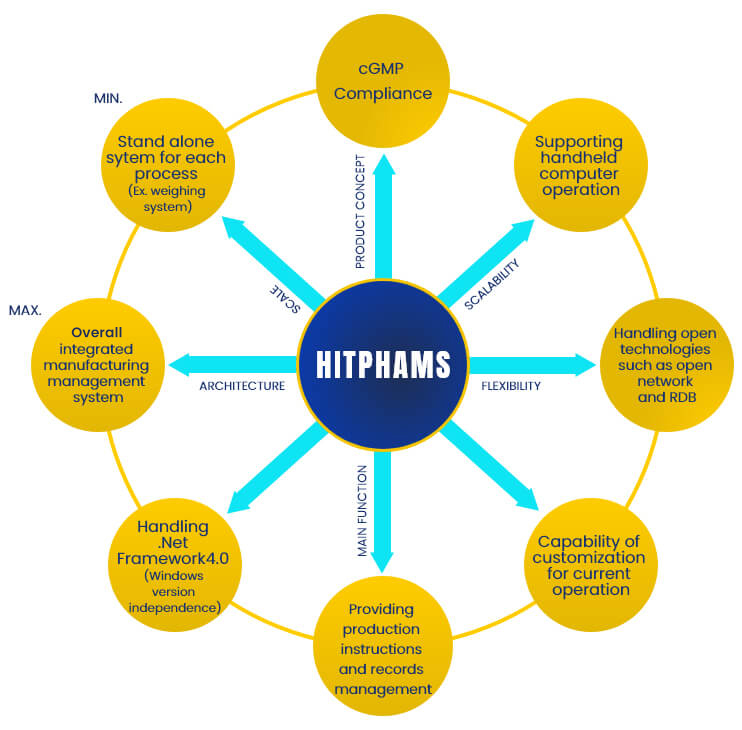
The main goal of HITPHAMS is to ensure the effective execution of manufacturing operations and improve production output. HITPHAMS Function is designed to meet ISA-95/ISO 62264 standards.
HITPHAMS exercises strict control over the production of high-quality, highly reliable pharmaceuticals within the framework of manufacturing instructions and manufacturing result records as designed for pharmaceuticals.
It helps to control the SOPs, confirm tasks, and execute approvals by the assigned representatives.
In HITPHAMS, users are empowered to create electronic batch reports, including attachments, using either tailor-made templates or established formats.
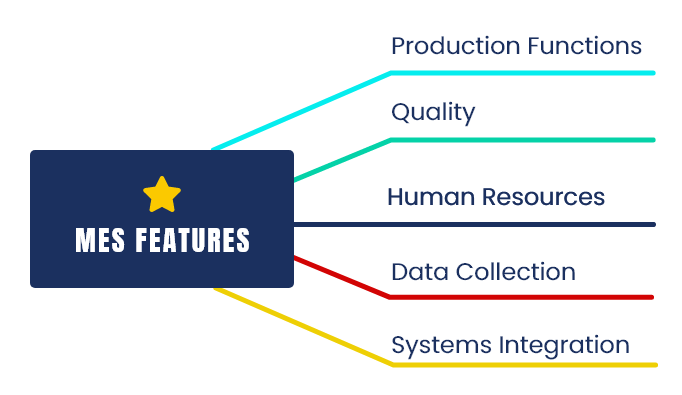
The Implementation typically covers the following functions
- Security
- Approval Management
- Calendar Management
- Error information handling
- Archival management
- Master Batch Record
- Workorder Management
- Manufacturing Management
- Inventory Management
- Quality Control
- Recipe management
- Weighing and dispensing
- In-process checks
- E-logbooks
- e-BMR & e-BPR
- ERP and other equipment integration
- Batch release
- Audit logs
- Deviation Management
Making your business ideas come true
The key benefits of HITPHAMS
- Compliance with latest global Good Manufacturing Principles (GMP) guidelines
- Strict Standard Operation Procedure (SOP) sequence implementation
- Promotion of accurate work
- Promotion of on-line and paperless operation
- CAPA and Complaint Handling.
- Improves the efficiency of creating analytical data records
- Reduce the risk of human error and minimize rework costs
- Tracking and traceability are ensured
- Paperless operations, data availability at a click of a button for surprise inspections
- Providing Quality and Data Integrity in Global Pharmaceutical Manufacturing.
- Offering the optimum system according to the scale
Strength of the OEM
- Well experienced pharmaceutical engineers to support the project.
- Vast experiences of implementing GMP related systems, eQMS, LIMS, SCADA, etc.
- No.1 IT service provider for Pharma Industry in Japan
- 70+ years History in Pharmaceutical Industry
- Hitachi has 22 years of experience in the Pharmaceutical MES area.
- Implementation of HITPHAMS: 140+ sites
- Hitachi hosts the most significant user conference in the pharmaceutical industry in Japan. Nearly 50 companies attend the annual meeting. The event provides an excellent opportunity to exchange ideas and discuss future requirements of HITPHAMS and solutions for compliance to “Good Practices.”